Many important applications of chemistry involve the transfer of electrons. Understanding the processes by which this occurs allows us to harnes the chemical energy generated by compbining dissimilar electrolytes. For example, this is how batteries are made. And in cases where the processes in the individial cells are reversible, recharchable batteries can be constructed.
In this part, you will simulate electroplating metal onto a key from 1.00 L of a 1.00 M solution of your chosen electroltyte. Base on this, you will determine the charge on the slectrolyte ion in solution.
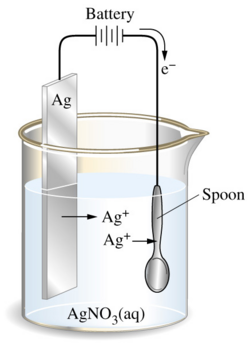
In this part, you will simulate the measurement of the electromotive force (EMF) generated under standard conditons at 25o for several pairs of half cells. You will then use this data to create a table of standard half-cell reduction potentials.
In this part, you will simulate the measurement of the EMF generated by similar half-cells using non-standard concetrations.
In this part, you will determine a value of Kf, the equilibrium constant for formation of a complex ion, using EMF measurments.