As a starting point, consider a handful of common polyatomic anions. These show up repeatedly in nature.
| some common polyatomic anions | |
| CN- | cyanide |
| N3- | azide |
| OCN- | cyanate |
| C2H3O2- | acetate |
| C2O4-2 | oxylate |
| CHO2- | formate |
Notice the endings for these ions. The names end in either -ide or -ate. These ending are very common in the names of anions. As we will see, the ending can be an important tipoff as to the oxidation number on the central atom in an anion.
Polyatomic oxyanions
The naming of oxyanions looks to be an overhelming task due to the large number of different ions which occur in nature. But fortunately, we have a very systematic method for naming these beasts. If you can simply see and learn the patterns, you will find the task of naming the ions quite simple.
Consider a few common ions found in nature:
| Polyatomic Oxyanions Containing: | |||||||||
| boron | carbon | nitrogen | oxygen | ||||||
| BO3-3 | orthoborate | CO3-2 | carbonate | NO3- | nitrate | O2-2 | peroxide | ||
| BO2- | metaborate | HCO3- | hydrogen carbonate (bicorbonate) |
NO2- | nitrite | OH- | hydroxide | ||
| B4O7-2 | tetraborate | ||||||||
| silicon | phosphorus | sulfur | chlorine | ||||||
| SiO4-4 | orthosilcate | PO4-3 | phosphate (orthophosphate) |
SO4-2 | sulfate | ClO4- | perchlorate | ||
| SiO3-2 | metasilcate | PO3-3 | phosphite | SO3-2 | sulfite | ClO3- | chlorate | ||
| Si2O5-2 | disilicate | HPO4-2 | hydrogen phosphate | S2O3-2 | thiosulfate | ClO2- | chlorite | ||
| H2PO4- | dihydrogen phosphate | HSO4- | hydrogen sulfate (bisulfate) |
ClO- | hypochlorite | ||||
| aresenic | selenium | bromine | |||||||
| AsO4-3 | orthoarsenate | SeO4-2 | selenate | BrO4- | perbromate | ||||
| AsO3- | metaarsenate | SeO3-2 | selenite | BrO3- | bromate | ||||
| HAsO4-2 | hydrogen arsenate | HSeO4- | hydrogen selenate | BrO2- | bromite | ||||
| H2AsO4- | dihydrogen arsenate | BrO- | hypobromite | ||||||
| tellurium | iodine | ||||||||
| TeO4-2 | tellurate | IO4- | periodate | ||||||
| TeO3-2 | tellurite | IO3- | iodate | ||||||
| IO2- | iodite | ||||||||
| IO- | hypoiodite | ||||||||
| chlorine | whatever | ||
| ClO4- | perchlorate | one extra O | per - ate |
| ClO3- | chlorate | normal # of O | - ate |
| ClO2- | chlorite | one fewer O | - ite |
| ClO- | hypochlorite | two fewer O | hypo - ite |
So, as an example, you can see that an ion with one extra oxygen atom is signified by adding the prefix "per-" to the name of the ion. This is even aparent in the naming of the ion peroxide! Another common ion in nature is the cyonate ion which has the formula OCN-.
Addition of H+ to an ion
Another important patterns include the addition of an H+ to the ion. This doesn't change the oxidation number on the central atom, but the name is changed by adding "hydrogen" to the name. Examples of this pattern can be found in the ions HCO3- (hydrogen carbonate) and HAsO4-2 (hydrogen arsenate). If two hydrogens are added (H2PO4- for example), the word dihydrogen is added to the name of the ion.
| some hydrogen added anions | |
| OH- | hydroxide (short for hydrogen oxide) |
| HSO4- | hydrogen sulfate (or bisulfate) |
| HCO3- | hydrogen carbonate (or bicarbonate) |
| H2PO4- | dihydrogen phosphate |
Older references will often use the prefix "bi-" to indicate the adition of an H+. To understand this, consider the salts sodium carbonate (Na2CO3) and sodium hydrogen carbonate (or sodium bicarbonate - NaHCO3). In sodium bicarbonate, the ratio of carbonate units to sodium ions is doubled compared to sodium carbonate.
meta- and ortho- prefixes
(Changing the number of oxygen atoms
without changing the oxidation number of the central atom)
Some oxyanions (such as those of boron, phosphorus and arsenic) can occur with different numbers of oxygen atoms, but the same oxidation number on the central atom. In these cases, the form with the larger number of oxygen atoms is given the prefix "ortho-" and the form with the smaller number of oxygen atoms is given the prefix "meta-". The pratical difference is that the "meta-" ions generally occur only under anhydrous conditions. And since water is very common on Earth, the "ortho-" forms are more commonly encountered. When no prefix is indicated, it can be safely assumed that the more common "ortho-" form is implied.
Replacement of oxygen with sulfur
Many ions exist which are just like the oxyanions shown above, except that one or more oxygen atoms has been preplaced by sulfur. In the table above, one such example is given - S2O3-2 - which is thiosulfate. The prefix "thio-" means sulfur. One way to think of the thiosulfate ion is a reguar sulfate ion in which one oxygen atom has been replaced by sulfur.
Many other examples of thiosubstituted ions exist in nature. Some examples include:
| some thio- ions | |
| AsS4-3 | thioarsenate |
| AsS3-3 | thioarsenite |
| CS3-2 | trithiocarbonate |
| SCN- | thiocyanate |
Thiosubstituted ions can be formed under geological conditions where oxygen isn't present. Notice that the pattern of -ate and -ite is retained in the ion name despite the substitution of sulfur.
Replacement of oxygen with fluorine
Another common substitution to find in ions is the substitution of fluorine for oxygen. Because oxygen has an oxidation number of -2 and fluorine has only an oxidation number of -1 in compounds, it generally takes two fluorine atoms per oxygen in the ion. With that in mind, the naming pattern is very similar.
| some fluo- ions | |
| SiF6-2 | fluosilicate |
| BF4- | fluoborate |
| PF6- | fluophosphate |
| GeF6-2 | fluogermanate |
Potassium fluosilicate is also know as the mineral hieritite. And potassium fluoborate is known as the mineral avogadrite. Many minerals have common names which are used by geologists. The geologist's names for ionic compounds sometimes seem more confusing that the chemist's names for the compounds, but keep in mind that geologists are often concerned not only with composition, but also crystaline structure.
Polymeric oxyanions
Some oxyanions are built from repeating structural units. They are common with central atoms which form neutral polymeric structures with oxygen linkages such as SiO2. Notice that in the polyatomic anions with an -ate ending, the central atoms ahve the same oxidation number as in the regular -ate ions. Consider some examples:
| polymeric oxyanions | |
| Si2O5-2 | disilicate |
| B4O7-2 | tetraborate |
Disilicate has a structure which looks like this:
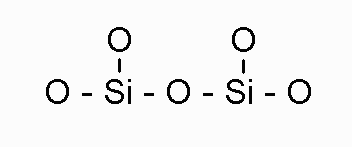 |
Oxyanions with metal centers
Several oxyanions occur in nature where the central atom is a metal atom. Some examples of these are:
| metal centered oxyanions | |
| WO4-2 | tungstate |
| MnO4-2 | manganate |
| MnO4- | permanganate |
| CrO4-2 | chromate |
| Cr2O7-2 | dichromate |
| MoO4-2 | molybdate |
| AuO2- | aurate |
Other polyatomic anions with metal centers
Many metal atoms can form anion complexes with chloride, cyanide or other species. Naming these species is particularly simple! Once again, it centers around the oxidation number on the central atom. A Greek prefix is used to give the number of ligand species in the ion and a roman numeral is used to indicate the oxidation number on the central atom. (CN- as a ligand is treated as an atom with and oxidation number of -1.) Sometimes these ions are not anions at all but have positive charges. These are typically ions which are complexed with ammonia (treated as an atom with an oxidation number of 0) or with insufficient negatively charged ligands to produce a nuetral species. A few examples will be helpful:
| metal complex anions and cations | |
| Fe(CN)6-4 | hexacyanoferrate (II) |
| Fe(CN)6-3 | hexacyanoferrate (III) |
| Cu(CN)4-3 | tetracyanocuprate (I) |
| NiCl4-2 | tetrachloronicklate (II) |
| Cu(NH3)4+2 | tetramincopper (II) |
| Ag(NH3)2+ | diaminsilver (I) |
A Note in Closing
Many, many other anions are found in nature. Only a handful are shown in this page to give an introduction to the patterns used in naming these ions. Keep these patterns in mind as you study your text and encounter ions in the world around you!
| Name the ion | ||||
Department of Chemistry and Biochemistry
California State University, East Bay
patrick.fleming@csueastbay.edu